Vitamin C Chemistry
From: Nutrition Almanac:
Vitamin C, also known as ascorbic acid, is a water-soluble nutrient. Although fairly stable in acid solution, it is normally the least stable of vitamins and is very sensitive to oxygen. Its potency can be lost through exposure to light, heat, and air, which stimulate the activity of oxidative enzymes.
Vitamin C or Ascorbic acid, is the enolic form of 3-oxo-L-gulofuranolactone. It can be prepared by synthesis from glucose, or extracted from plant sources such as rose hips, blackcurrants or citrus fruits. It is easily oxidised in air. It is essential for the formation of collagen and intercellular material, bone and teeth and for the healing of wounds. It helps maintain elasticity of the skin, aids the absorption of iron and improves resistance to infection. It is used in the treatment of scurvy. May prevent the occurrence and development of cancer.
Man is one of the few mammals unable to manufacture ascorbic acid in his liver.
Good sources of Vitamin C are Broccoli, Brussels sprouts, cauliflower, cabbage, green leafy vegetables, red peppers, chilies, watercress, parsley, blackcurrants, strawberries, kiwi fruit, guavas, citrus fruit. (Food Values)
Also used as a photographic developing agent in alkaline solution.
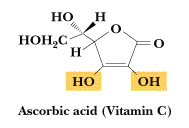
m.p. 190-192 oC., C6H8O6
Our thanks to Karl Harrison of the University of Oxford for providing us the description, diagram and 3D rendering shown here. Please visit his site, Molecules of the Month for many other descriptions and 3D renderings.

